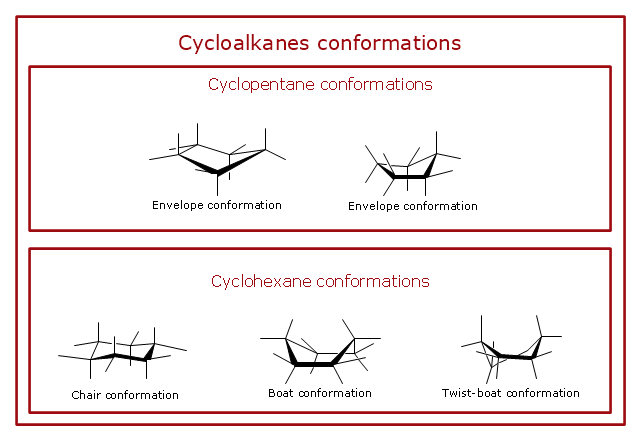
"A cyclohexane conformation is any of several three-dimensional shapes that a cyclohexane molecule can assume while maintaining the integrity of its chemical bonds.
The internal angles of a flat regular hexagon are 120°, while the preferred angle between successive bonds in a carbon chain is about 109.5°, the tetrahedral angle. Therefore the cyclohexane ring tends to assume certain non-planar (warped) conformations, which have all angles closer to 109.5° and therefore a lower strain energy than the flat hexagonal shape. The most important shapes are called chair, half-chair, boat, and twist-boat. The molecule can easily switch between these conformations, and only two of them - chair and twist-boat - can be isolated in pure form.
Cyclohexane conformations have been extensively studied in organic chemistry because they are the classical example of conformational isomerism and have noticeable influence on the physical and chemical properties of cyclohexane." [Cyclohexane conformation. Wikipedia]
The chemical drawing example "Cycloalkanes conformations" was created using the ConceptDraw PRO diagramming and vector drawing software extended with the Chemistry solution from the Science and Education area of ConceptDraw Solution Park.
The internal angles of a flat regular hexagon are 120°, while the preferred angle between successive bonds in a carbon chain is about 109.5°, the tetrahedral angle. Therefore the cyclohexane ring tends to assume certain non-planar (warped) conformations, which have all angles closer to 109.5° and therefore a lower strain energy than the flat hexagonal shape. The most important shapes are called chair, half-chair, boat, and twist-boat. The molecule can easily switch between these conformations, and only two of them - chair and twist-boat - can be isolated in pure form.
Cyclohexane conformations have been extensively studied in organic chemistry because they are the classical example of conformational isomerism and have noticeable influence on the physical and chemical properties of cyclohexane." [Cyclohexane conformation. Wikipedia]
The chemical drawing example "Cycloalkanes conformations" was created using the ConceptDraw PRO diagramming and vector drawing software extended with the Chemistry solution from the Science and Education area of ConceptDraw Solution Park.
Organic Chemistry Symbols
ConceptDraw PRO diagramming and vector drawing software extended with Chemistry solution from the Science and Education area of ConceptDraw Solution Park is effective for drawing various organic chemistry schemes, diagrams, illustrations thanks to the included collection of predesigned organic chemistry symbols.
 Chemistry
Chemistry
This solution extends ConceptDraw PRO software with samples, template and libraries of vector stencils for drawing the Chemistry Illustrations for science and education.
- Planar Hexagon Conformation Of Cyclohexane
- Cycloalkanes conformations | Cycloalkanes conformations ...
- Cyclohexane Conformations
- Cycloalkanes conformations | Cyclohexane Chair Half Chair
- Conformations - Vector stencils library | Cycloalkanes conformations ...
- Cycloalkanes conformations | Design elements - Conformations ...
- Cycloalkanes conformations | Half Chair Conformer Of Cyclopentane
- Cycloalkanes conformations | Sunrooms - Vector stencils library ...
- Structure Of Half Chair Conformation Of Cyclohexane
- Conformations - Vector stencils library | Watercraft - Vector stencils ...
- Plane geometry - Vector stencils library | Cycloalkanes ...
- Conformations - Vector stencils library | Design elements ...
- Sofas and chairs - Vector stencils library | Office furniture - Vector ...
- Conformations - Vector stencils library | Watercraft - Vector stencils ...
- Organic Chemistry Symbols | Chemistry Symbols and Meanings ...
- Organic Chemistry Symbols | Chemistry Drawing Software ...
- Biology Drawing Software | Biology Drawing | Biology | Biochemical ...
- Plane geometry - Vector stencils library | Vicious circle - Crystal ...
- Solid geometry
- Sofas and chairs - Vector stencils library | Office furniture - Vector ...