Pyramid Chart Examples
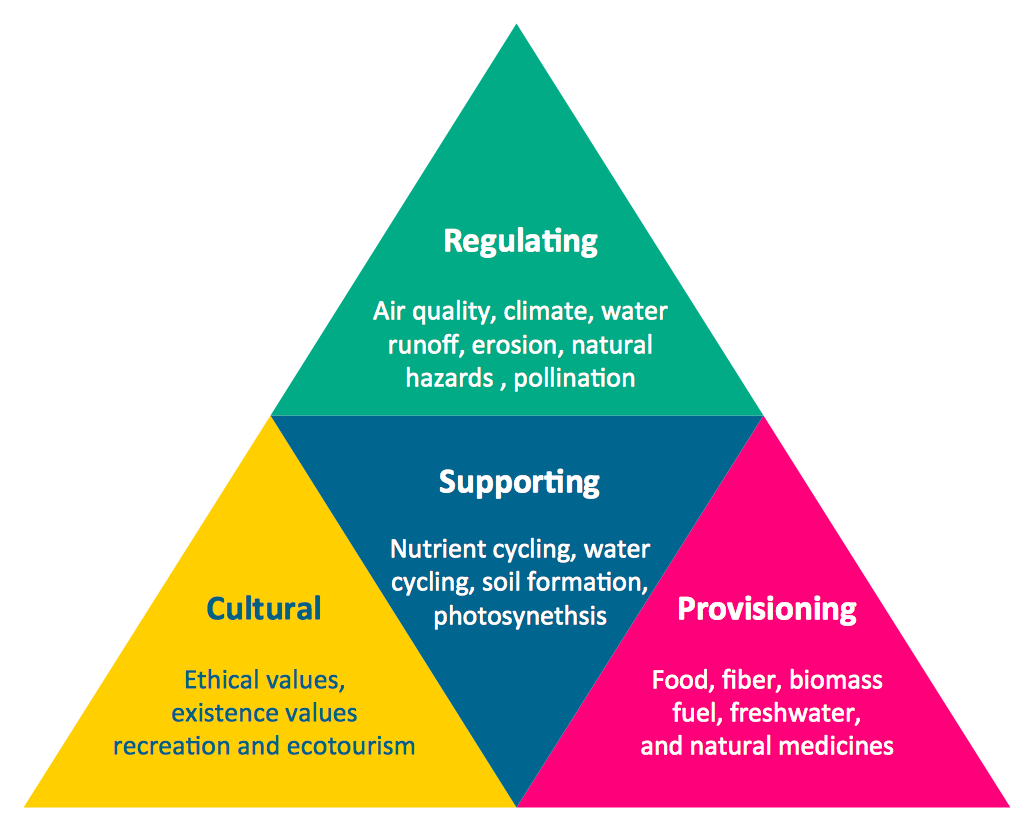
Pyramid Charts and Triangle Diagrams are used to visually structure the topics and progressively order the quantitative data. They allow to illustrate hierarchical structure of the topics, proportional, interconnected and containment relations among the topics. The multilevel Pyramids and Triangle diagrams are constructed oriented up or down and divided into several horizontal slices. They are effectively used to represent marketing strategies, social strategies, information systems, market value, etc., to illustrate presentations, websites, documents, reports in business, finances, sales, management, marketing, media, training, consulting, and many other fields. To maximize the efficiency in drawing the Pyramid Charts, use the ConceptDraw DIAGRAM diagramming and vector drawing software extended with Pyramid Diagrams solution from Marketing area, which contains the set of Pyramid Chart examples, samples, templates and vector design elements of triangular diagrams and pyramids with different quantity of levels for various needs."In colloquial language and fictional literature, seduction is the process of deliberately enticing a person, to lead astray, as from duty, rectitude, or the like; to corrupt, to persuade or induce to engage in sexual behaviour." [Seduction. Wikipedia]
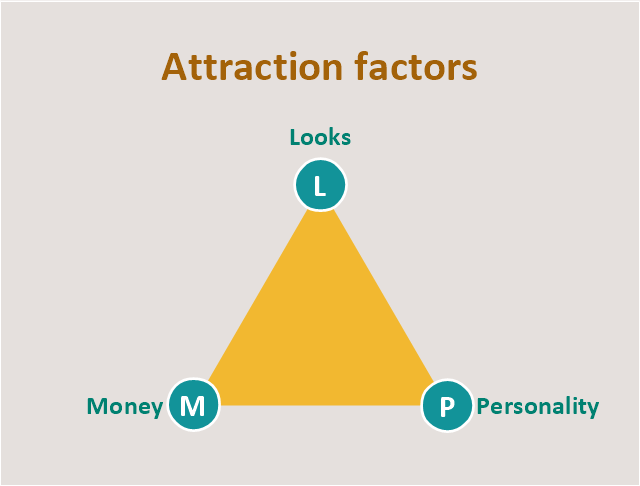
This Dna model of seduction triangular diagram was redesigned using the ConceptDraw PRO diagramming and vector drawing software from Commons Wikimedia file Dnamodel.jpg. [commons.wikimedia.org/ wiki/ File:Dnamodel.jpg]
This triangle diagram example "Dna model of seduction" is included in the Pyramid Diagrams solution from the Marketing area of ConceptDraw Solution Park.
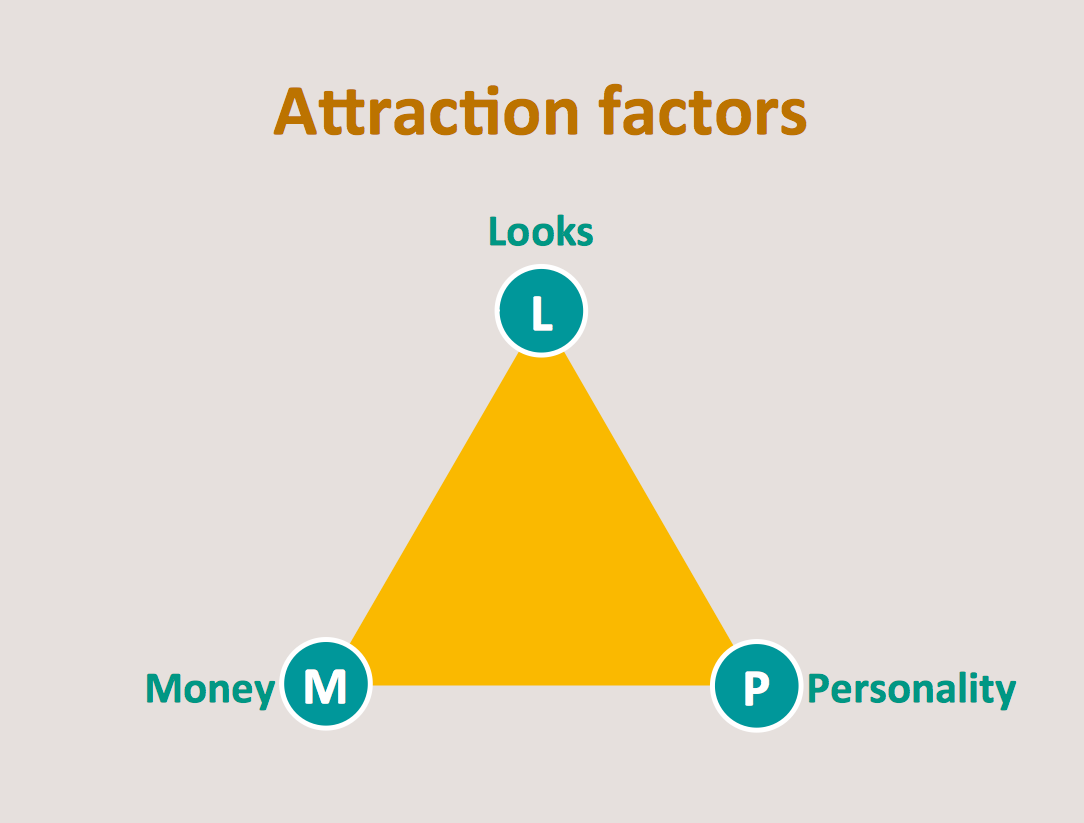
This Dna model of seduction triangular diagram was redesigned using the ConceptDraw PRO diagramming and vector drawing software from Commons Wikimedia file Dnamodel.jpg. [commons.wikimedia.org/ wiki/ File:Dnamodel.jpg]
This triangle diagram example "Dna model of seduction" is included in the Pyramid Diagrams solution from the Marketing area of ConceptDraw Solution Park.
 Pyramid Diagrams
Pyramid Diagrams
Pyramid Diagrams solution extends ConceptDraw DIAGRAM software with templates, samples and library of vector stencils for drawing the marketing pyramid diagrams.
Diagram of a Pyramid
Pyramid diagram is a convenient way of representing data hierarchical structure and visualization relationships between hierarchy levels. You need create the diagram of a pyramid? ConceptDraw diagramming and vector drawing software supplied with Pyramid Diagrams Solution from the Marketing Area of ConceptDraw Solution Park is the best tool for drawing attractive and successful diagram of a pyramid.Competitor Analysis
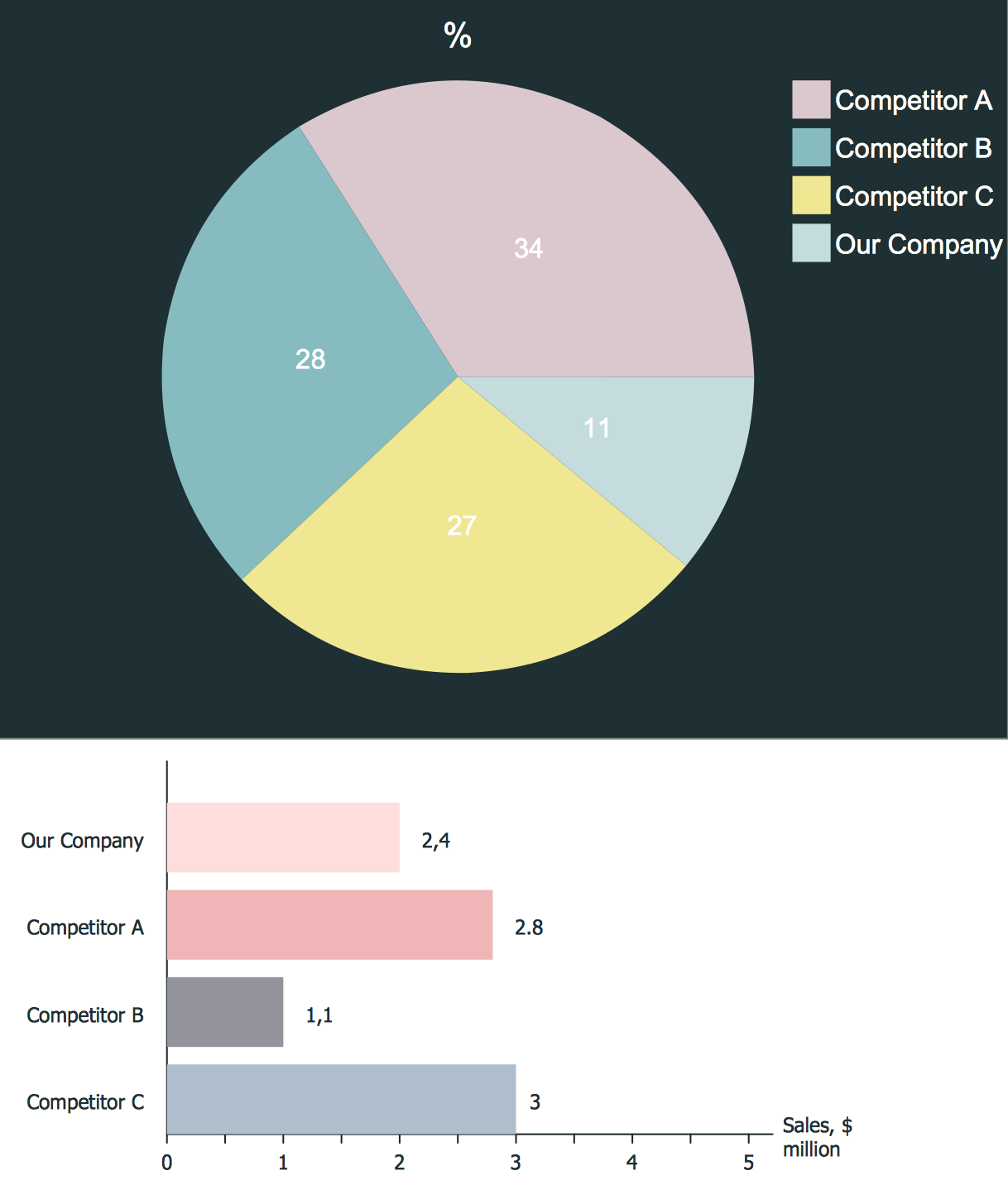
Competitor analysis is a first and obligatory step in elaboration the proper corporate marketing strategy and creating sustainable competitive advantage. Use powerful opportunities of numerous solutions from ConceptDraw Solution Park for designing illustrative diagrams, charts, matrices which are necessary for effective competitor analysis.Circular Diagram
Circular Diagram is a type of diagram widely used in marketing and economics for visualization information in a clear and visual form. ConceptDraw DIAGRAM diagramming and vector drawing software offers the useful tools of the Target and Circular Diagrams solution from the Marketing area of ConceptDraw Solution Park for effective drawing a Circular Diagram of any complexity and design.
 Healthcare Management Workflow Diagrams
Healthcare Management Workflow Diagrams
Healthcare Management Workflow Diagrams solution contains large set of colorful samples and libraries with predesigned vector pictograms and symbols of health, healthcare equipment, medical instruments, pharmaceutical tools, transport, medication, departments of healthcare organizations, the medical icons of people and human anatomy, as well as the predesigned flowchart objects, connectors and arrows, which make it the best for designing clear and comprehensive Medi?al Workflow Diagrams and Block Diagrams, Healthcare Management Flowcharts and Infographics, Healthcare Workflow Diagram, for depicting the healthcare workflow and clinical workflows in healthcare, for making the workflow analysis healthcare and healthcare workflow management.
The vector stencils library "Education pictograms" contains 128 education pictograms. Use this flat icon set to design your educational infogram in ConceptDraw PRO diagramming and vector drawing software.
The vector stencils library "Education pictograms" is included in the Education Infographics solution from the Business Infographics area of ConceptDraw Solution Park.
The vector stencils library "Education pictograms" is included in the Education Infographics solution from the Business Infographics area of ConceptDraw Solution Park.
 Pharmacy Illustrations
Pharmacy Illustrations
Pharmacy Illustrations solution with improbable quantity of predesigned vector objects and icons of pharmacy symbols, medical and health products, pharmacy images of drugstore products, pharmacy clipart of medication tools, pharmacy logo, and other pharmacy pictures is the best for designing the pharmacy illustrations of varied kinds, pharmacy and medical diagrams and schematics, for making the presentation slides and posters on the medical, pharmacy, pharmacology and pharmaceutical thematics, for designing the illustrative materials about ways of prevention diseases and also treatment them, for creation colorful illustrations helpful in newborn and baby care, the infographics and collages to be presented at the premises of medical establishments and during the lectures at the medical education institutions, also on the billboards and in other advertising materials.
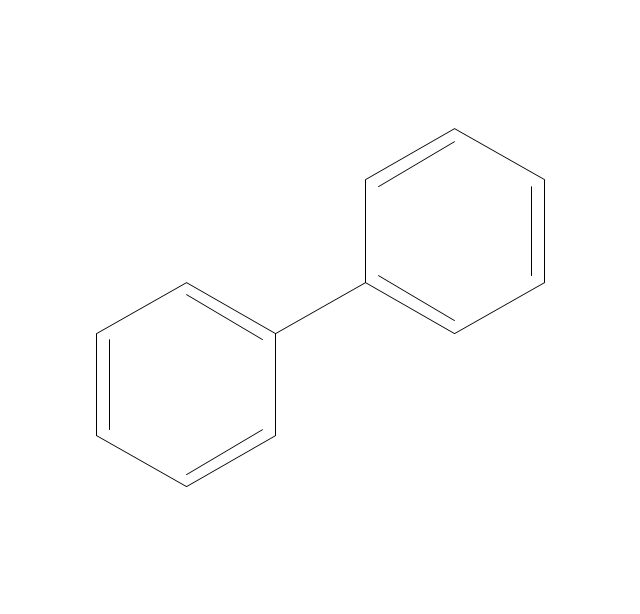
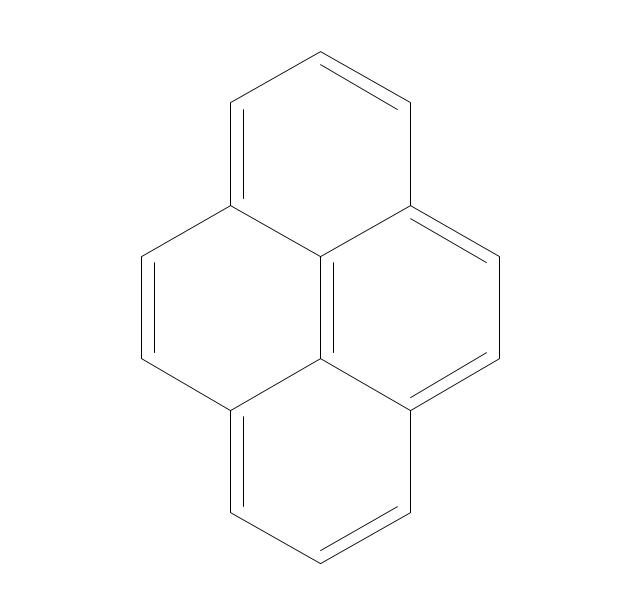
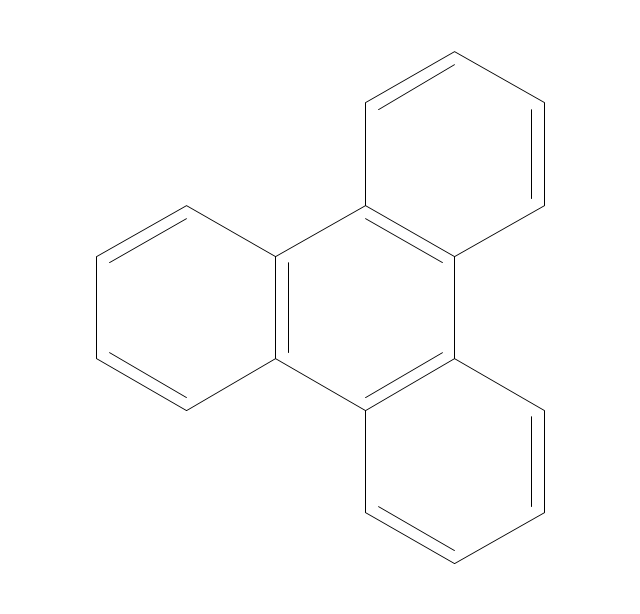
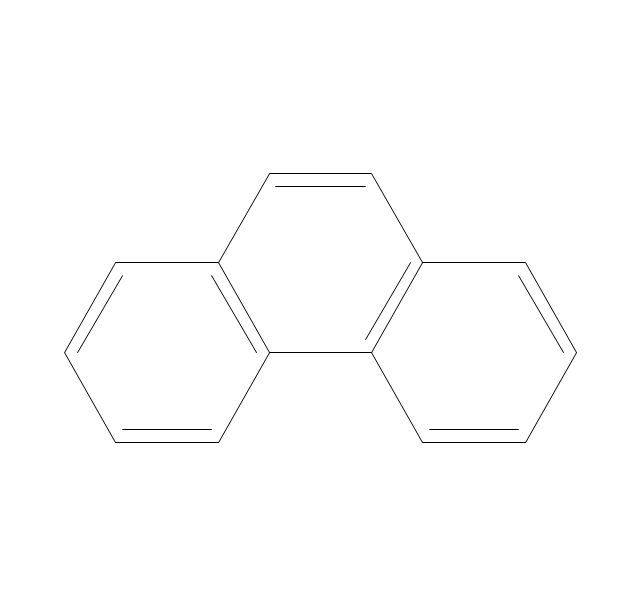
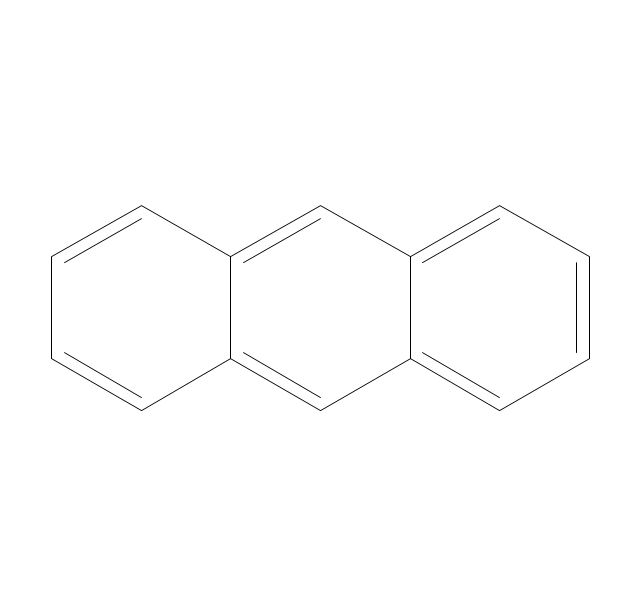
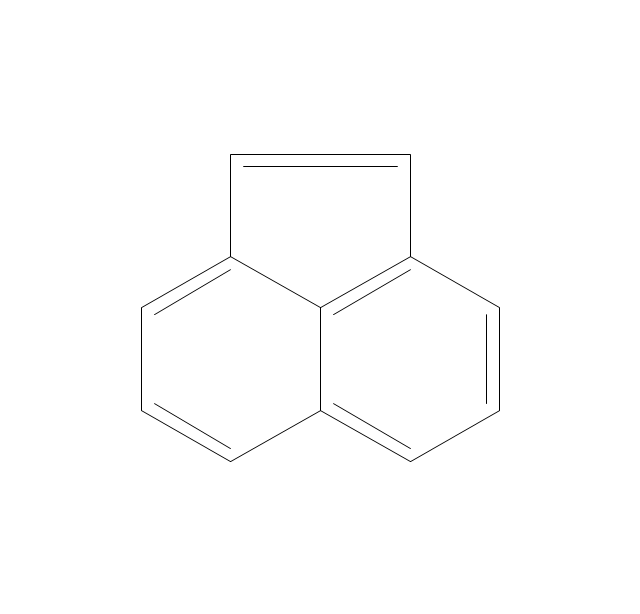
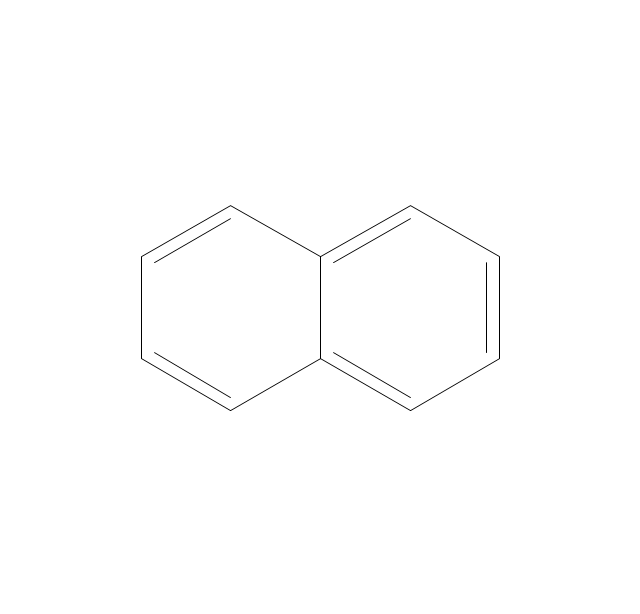
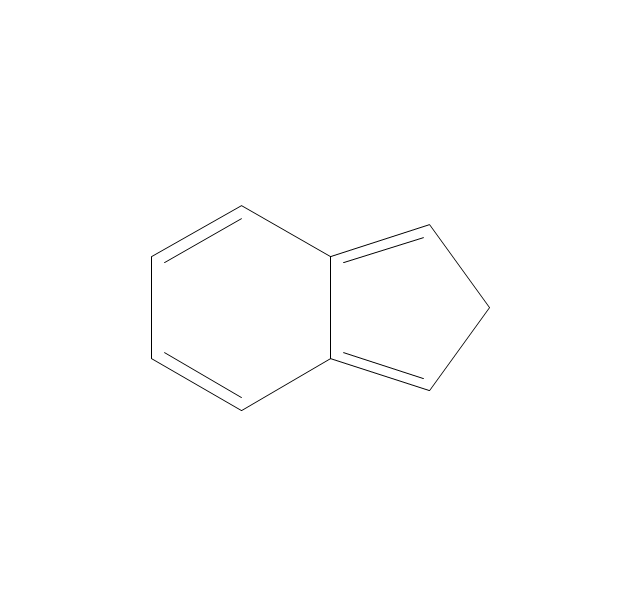
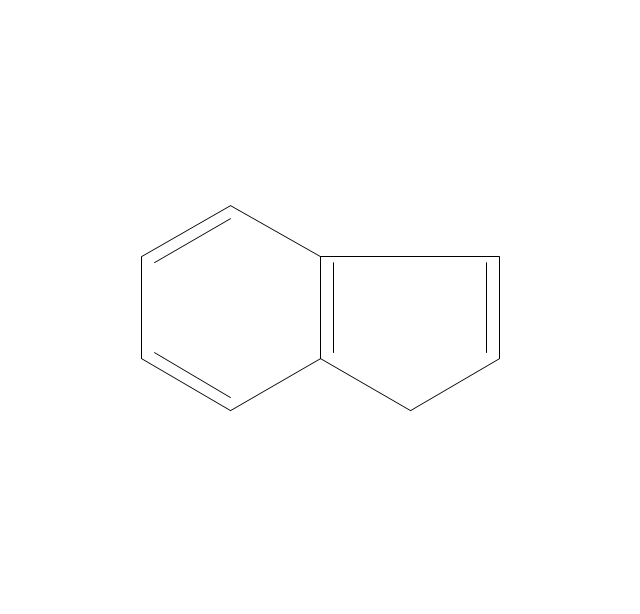
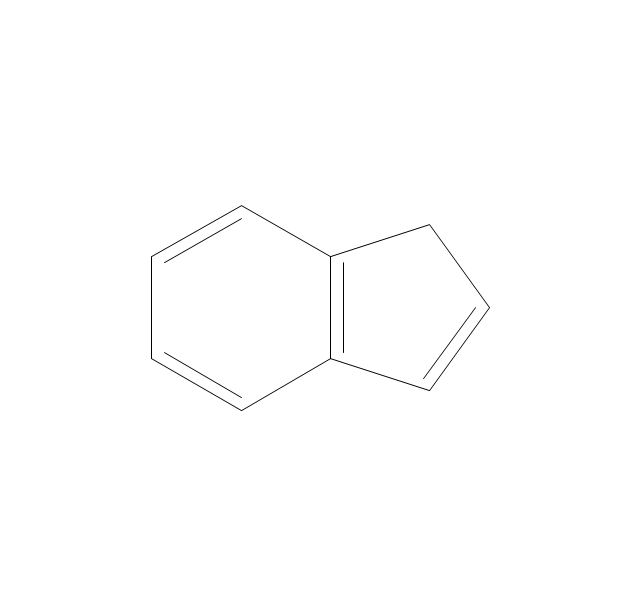
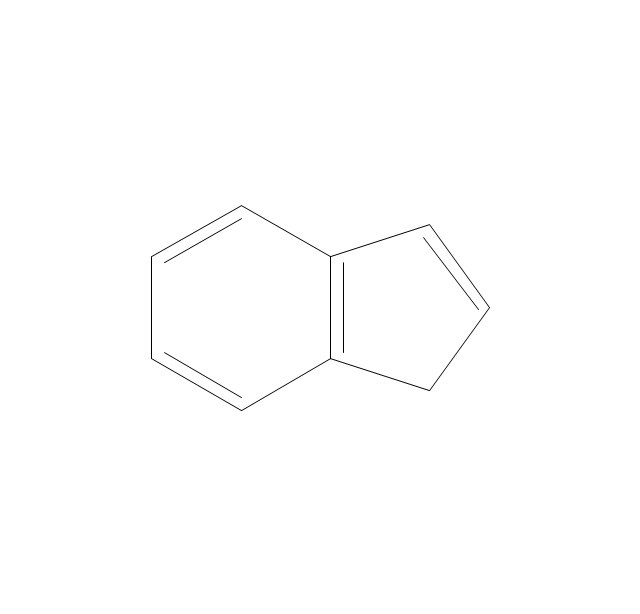
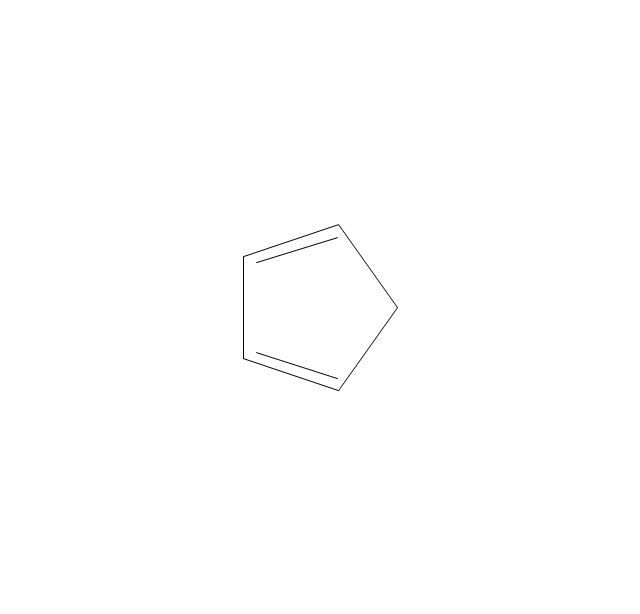
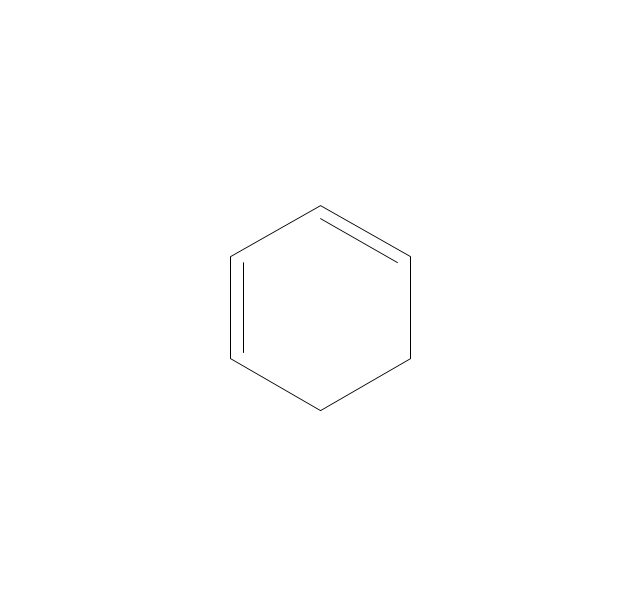
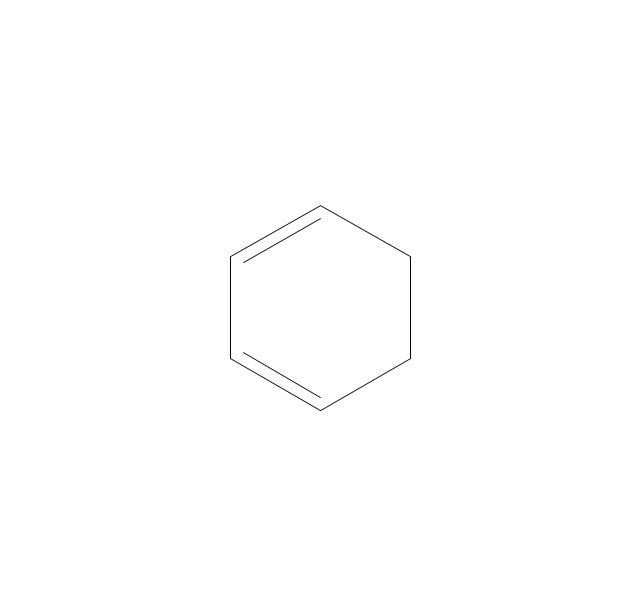
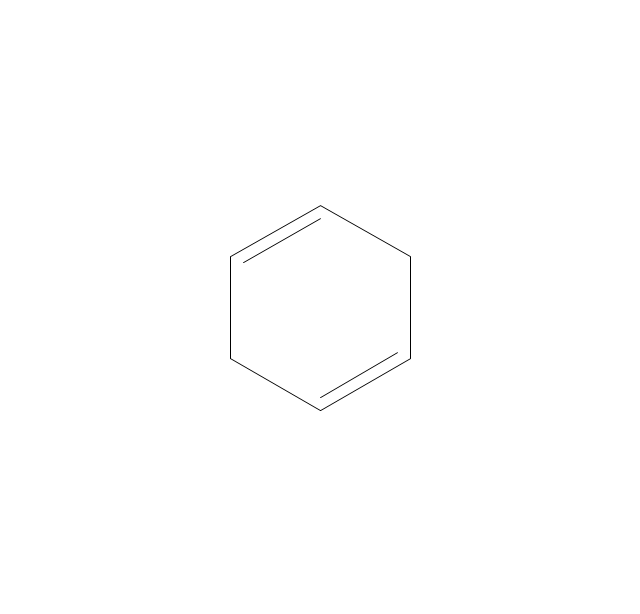
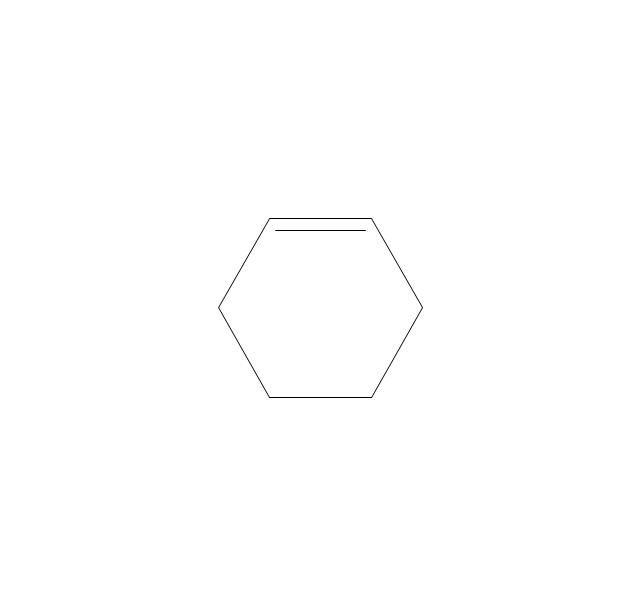
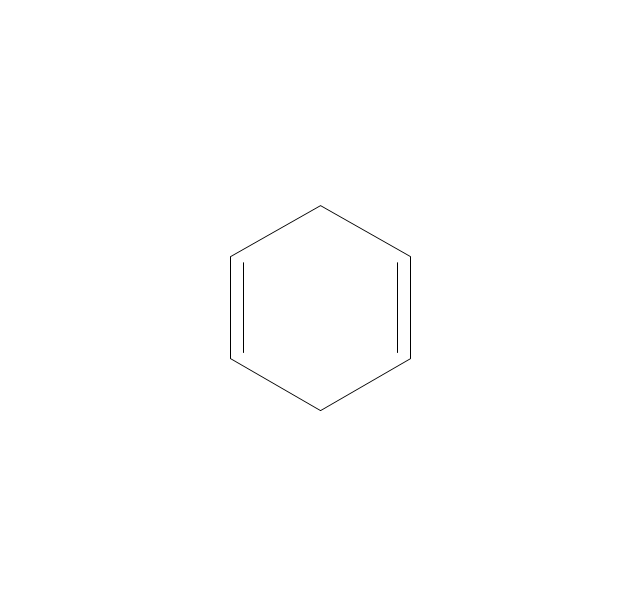
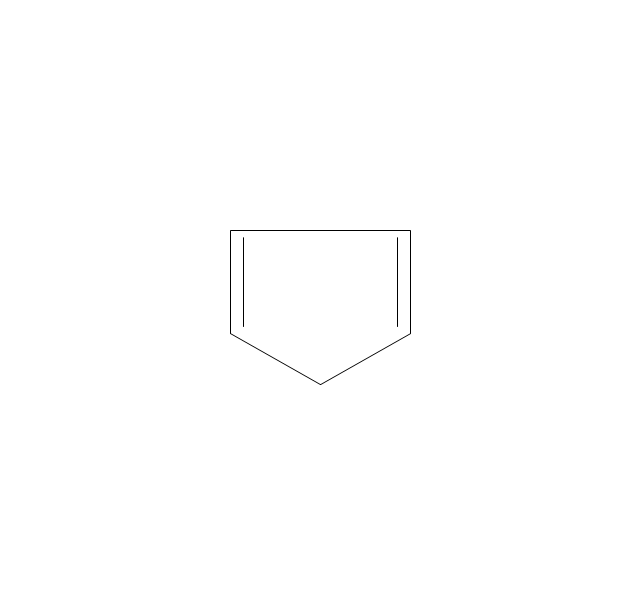
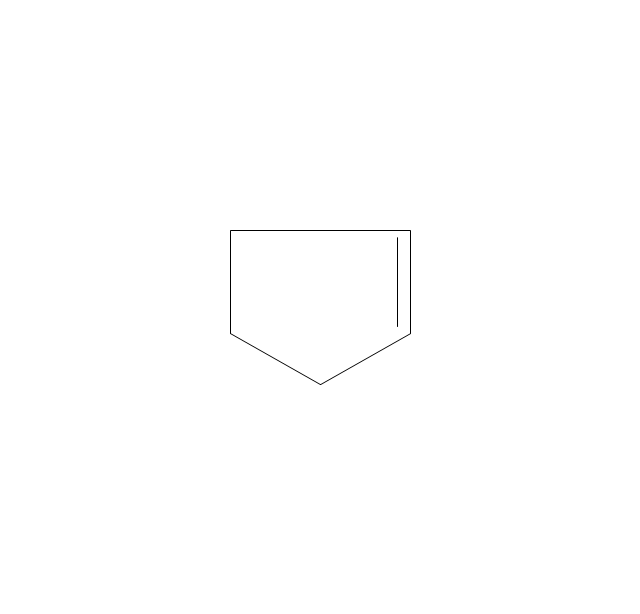
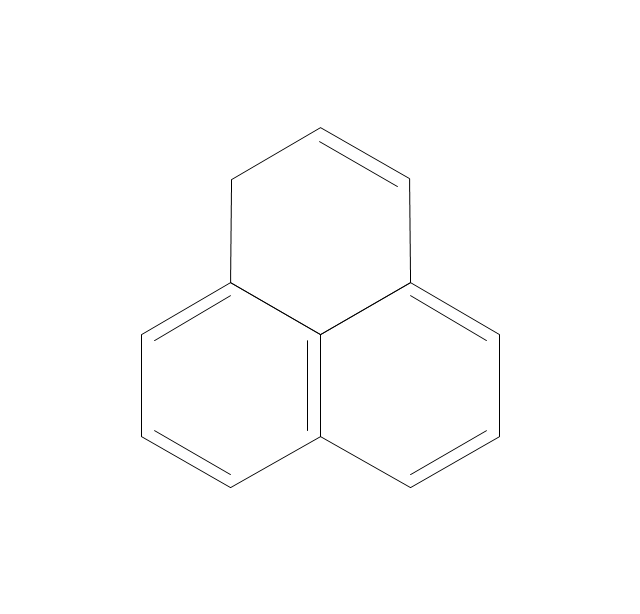
The vector stencils library "Aromatics" contains 23 symbols of aromatic rings for chemical drawing of molecular structural formulas and reaction mechanism schemes in organic chemistry.
"In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected by the stabilization of conjugation alone. ... Aromaticity can also be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-bonded to one another. These bonds may be seen as a hybrid of a single bond and a double bond, each bond in the ring identical to every other. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by Kekulé (see History section below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to give rise to six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization. ... Types of aromatic compounds. The overwhelming majority of aromatic compounds are compounds of carbon, but they need not be hydrocarbons. 1. Neutral homocyclics. Benzene, as well as most other annulenes (cyclodecapentaene excepted) with the formula CnHn where n is an even number, such as cyclotetradecaheptaene. 2. Heterocyclics. In heterocyclic aromatics (heteroaromats), one or more of the atoms in the aromatic ring is of an element other than carbon. This can lessen the ring's aromaticity, and thus (as in the case of furan) increase its reactivity. Other examples include pyridine, pyrazine, imidazole, pyrazole, oxazole, thiophene, and their benzannulated analogs (benzimidazole, for example). 3. Polycyclics. Polycyclic aromatic hydrocarbons are molecules containing two or more simple aromatic rings fused together by sharing two neighboring carbon atoms (see also simple aromatic rings). Examples are naphthalene, anthracene, and phenanthrene. 4. Substituted aromatics. Many chemical compounds are aromatic rings with other functional groups attached. Examples include trinitrotoluene (TNT), acetylsalicylic acid (aspirin), paracetamol, and the nucleotides of DNA. 5. Atypical aromatic compounds. Aromaticity is found in ions as well: the cyclopropenyl cation (2e system), the cyclopentadienyl anion (6e system), the tropylium ion (6e), and the cyclooctatetraene dianion (10e). Aromatic properties have been attributed to non-benzenoid compounds such as tropone. Aromatic properties are tested to the limit in a class of compounds called cyclophanes. A special case of aromaticity is found in homoaromaticity where conjugation is interrupted by a single sp³ hybridized carbon atom. When carbon in benzene is replaced by other elements in borabenzene, silabenzene, germanabenzene, stannabenzene, phosphorine or pyrylium salts the aromaticity is still retained. Aromaticity also occurs in compounds that are not carbon-based at all. Inorganic 6-membered-ring compounds analogous to benzene have been synthesized. Hexasilabenzene (Si6H6) and borazine (B3N3H6) are structurally analogous to benzene, with the carbon atoms replaced by another element or elements. In borazine, the boron and nitrogen atoms alternate around the ring." [Aromaticity. Wikipedia]
The organic compound structural formulas example "Aromatics - Vector stencils library" was created using the ConceptDraw PRO software extended with the Chemistry solution from the Science and Education area of ConceptDraw Solution Park.
"In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected by the stabilization of conjugation alone. ... Aromaticity can also be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-bonded to one another. These bonds may be seen as a hybrid of a single bond and a double bond, each bond in the ring identical to every other. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by Kekulé (see History section below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to give rise to six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization. ... Types of aromatic compounds. The overwhelming majority of aromatic compounds are compounds of carbon, but they need not be hydrocarbons. 1. Neutral homocyclics. Benzene, as well as most other annulenes (cyclodecapentaene excepted) with the formula CnHn where n is an even number, such as cyclotetradecaheptaene. 2. Heterocyclics. In heterocyclic aromatics (heteroaromats), one or more of the atoms in the aromatic ring is of an element other than carbon. This can lessen the ring's aromaticity, and thus (as in the case of furan) increase its reactivity. Other examples include pyridine, pyrazine, imidazole, pyrazole, oxazole, thiophene, and their benzannulated analogs (benzimidazole, for example). 3. Polycyclics. Polycyclic aromatic hydrocarbons are molecules containing two or more simple aromatic rings fused together by sharing two neighboring carbon atoms (see also simple aromatic rings). Examples are naphthalene, anthracene, and phenanthrene. 4. Substituted aromatics. Many chemical compounds are aromatic rings with other functional groups attached. Examples include trinitrotoluene (TNT), acetylsalicylic acid (aspirin), paracetamol, and the nucleotides of DNA. 5. Atypical aromatic compounds. Aromaticity is found in ions as well: the cyclopropenyl cation (2e system), the cyclopentadienyl anion (6e system), the tropylium ion (6e), and the cyclooctatetraene dianion (10e). Aromatic properties have been attributed to non-benzenoid compounds such as tropone. Aromatic properties are tested to the limit in a class of compounds called cyclophanes. A special case of aromaticity is found in homoaromaticity where conjugation is interrupted by a single sp³ hybridized carbon atom. When carbon in benzene is replaced by other elements in borabenzene, silabenzene, germanabenzene, stannabenzene, phosphorine or pyrylium salts the aromaticity is still retained. Aromaticity also occurs in compounds that are not carbon-based at all. Inorganic 6-membered-ring compounds analogous to benzene have been synthesized. Hexasilabenzene (Si6H6) and borazine (B3N3H6) are structurally analogous to benzene, with the carbon atoms replaced by another element or elements. In borazine, the boron and nitrogen atoms alternate around the ring." [Aromaticity. Wikipedia]
The organic compound structural formulas example "Aromatics - Vector stencils library" was created using the ConceptDraw PRO software extended with the Chemistry solution from the Science and Education area of ConceptDraw Solution Park.
- Pyramid Chart Examples | Dna model of seduction - Triangular ...
- Dna model of seduction - Triangular diagram | Pyramid Chart ...
- Dna model of seduction - Triangular diagram | Education pictograms ...
- Education pictograms - Vector stencils library | Dna model of ...
- Pyramid Chart Examples | Pyramid Diagrams | Brand Dna Pyramid
- Brand Dna Examples
- Organizational culture - Triangle diagram | Dna model of seduction ...
- Dna Replication Flow Chart
- Replication Of Dna Flow Chart