The vector stencils library "Chemical elements" contains 118 icon symbols of chemical elements.
Use these shapes for drawing atoms, structural formulas of inorganic and organic molecules and ions, and schemes of chemical reaction mechanisms in the ConceptDraw PRO diagramming and vector drawing software extended with the Chemistry solution from the Science and Education area of ConceptDraw Solution Park.
www.conceptdraw.com/ solution-park/ science-education-chemistry
Use these shapes for drawing atoms, structural formulas of inorganic and organic molecules and ions, and schemes of chemical reaction mechanisms in the ConceptDraw PRO diagramming and vector drawing software extended with the Chemistry solution from the Science and Education area of ConceptDraw Solution Park.
www.conceptdraw.com/ solution-park/ science-education-chemistry
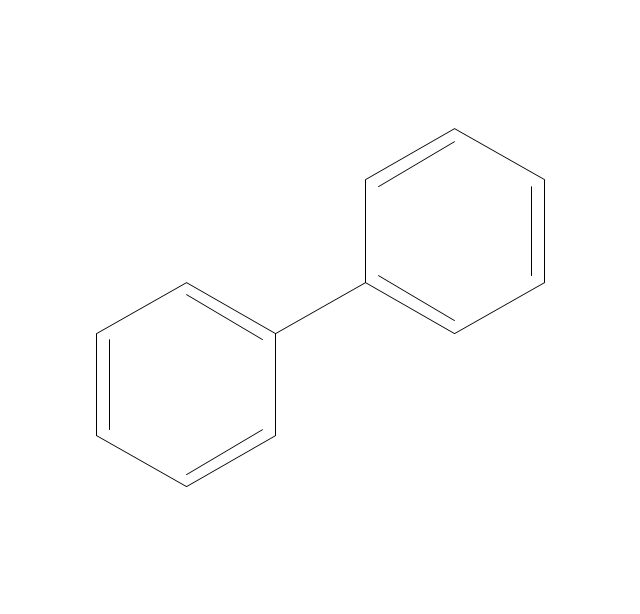
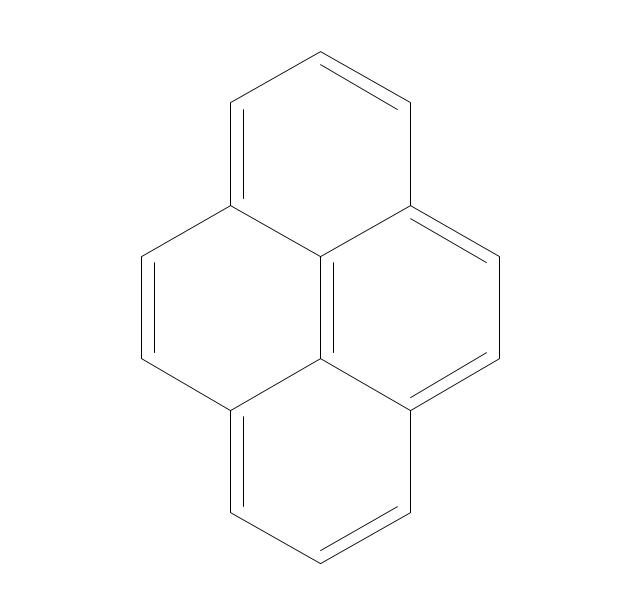
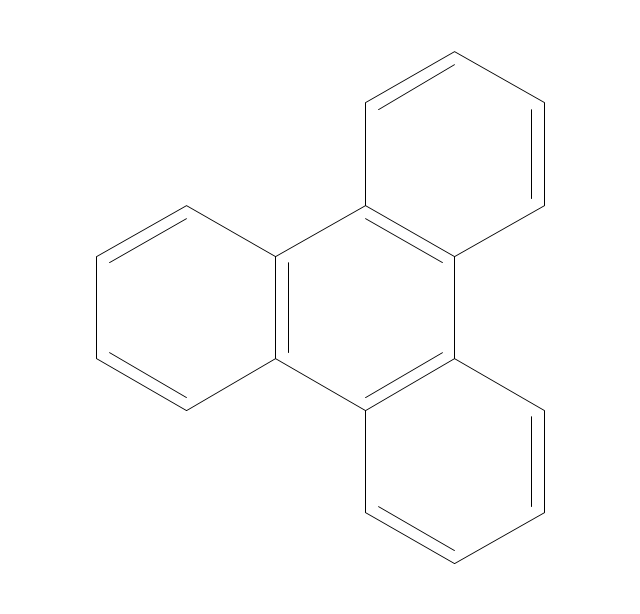
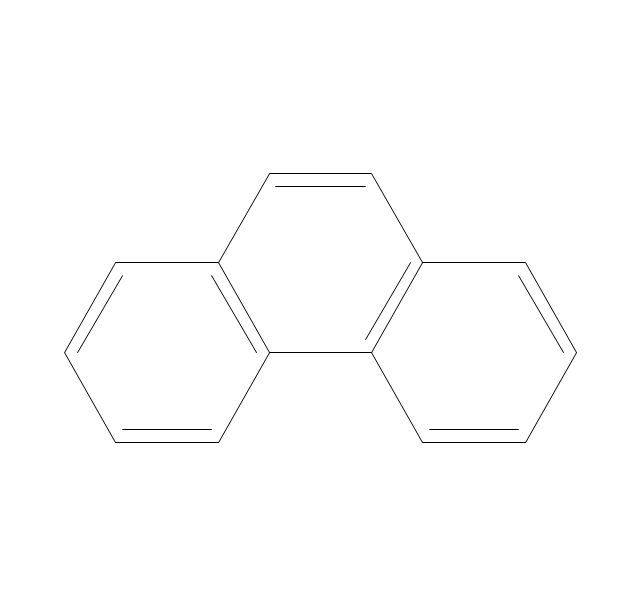
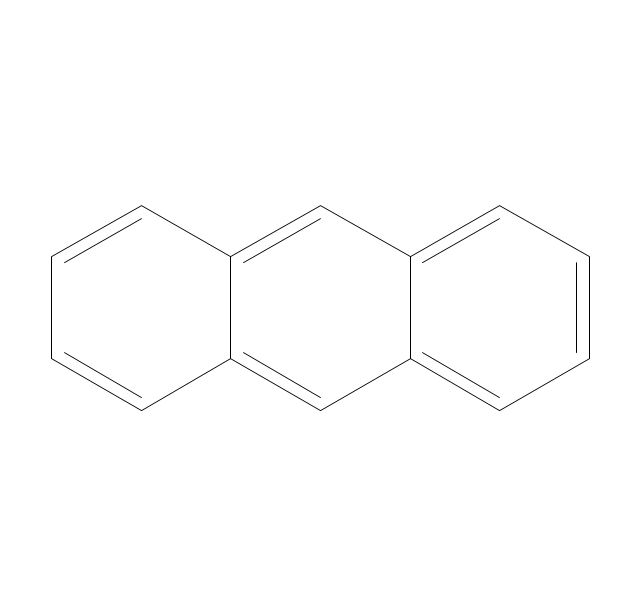
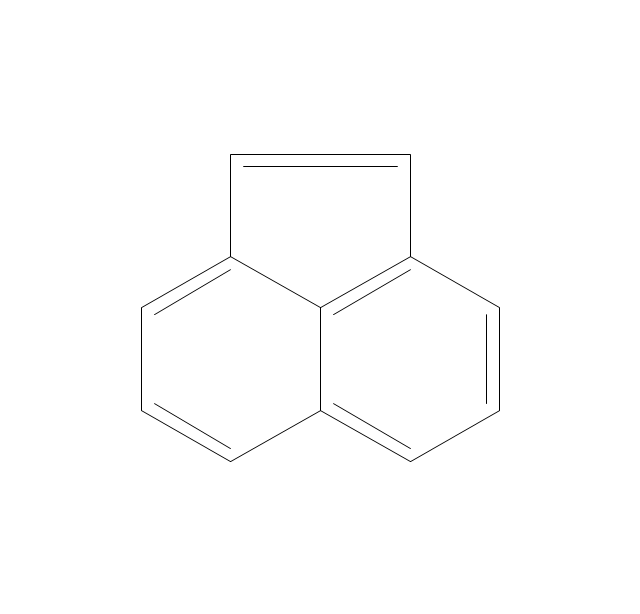
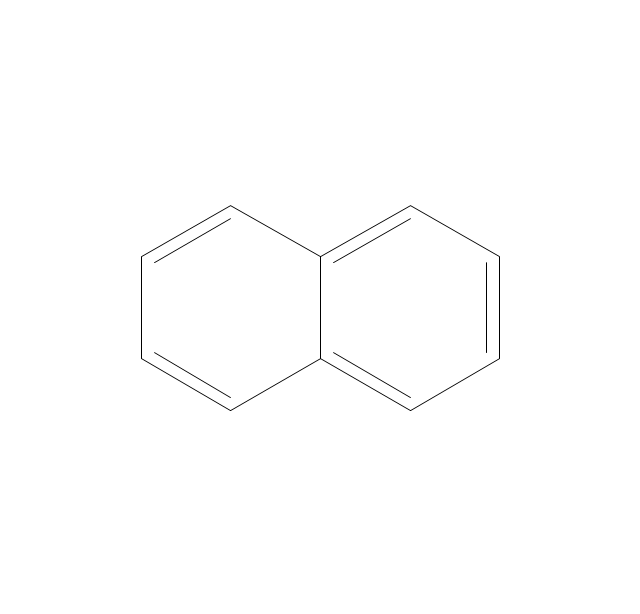
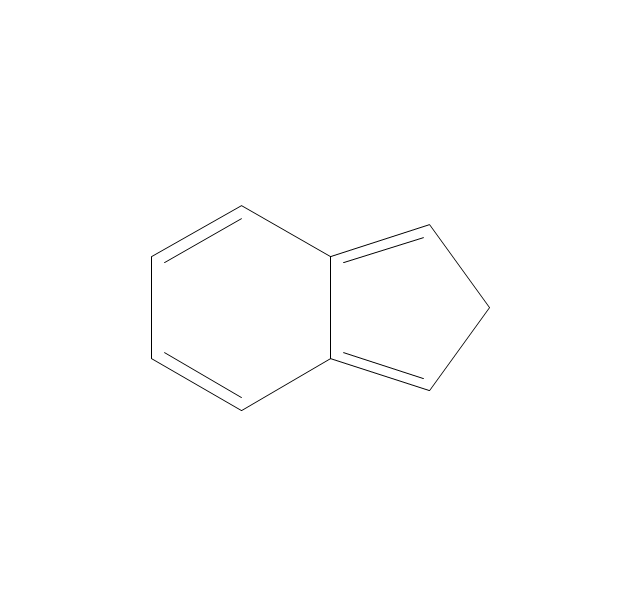
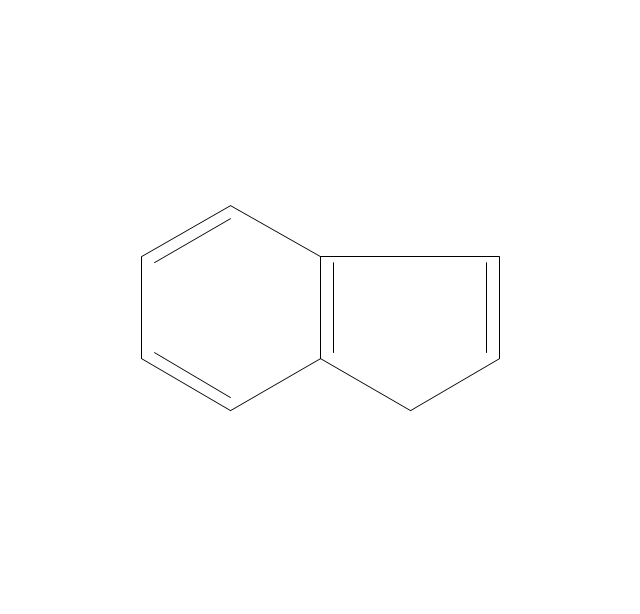
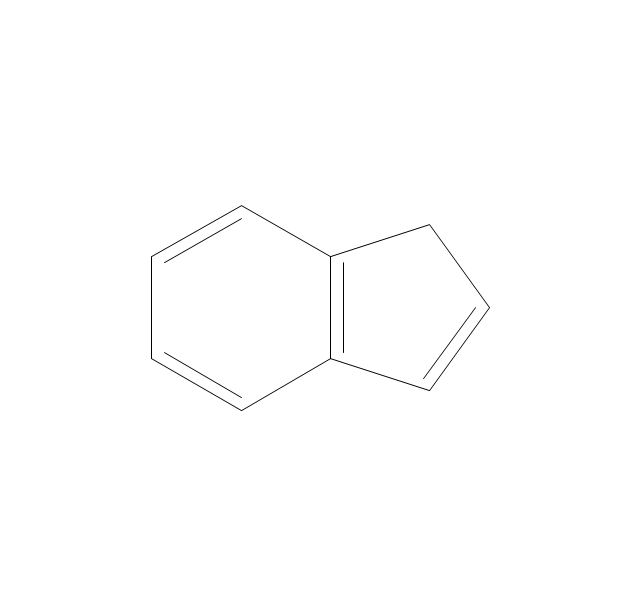
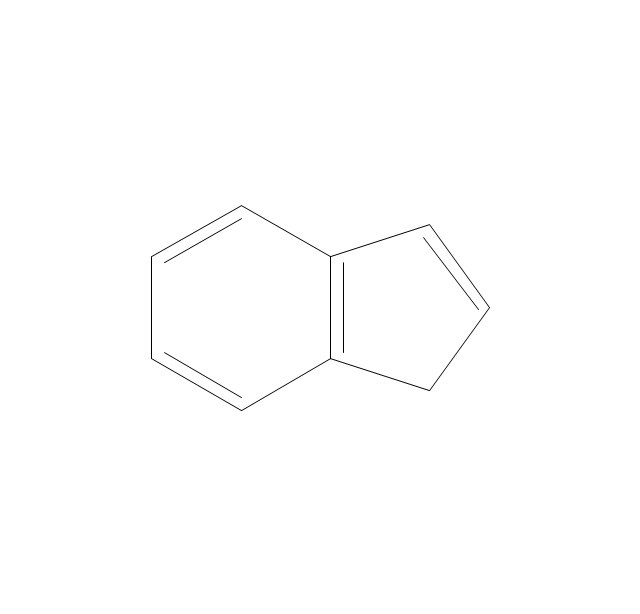
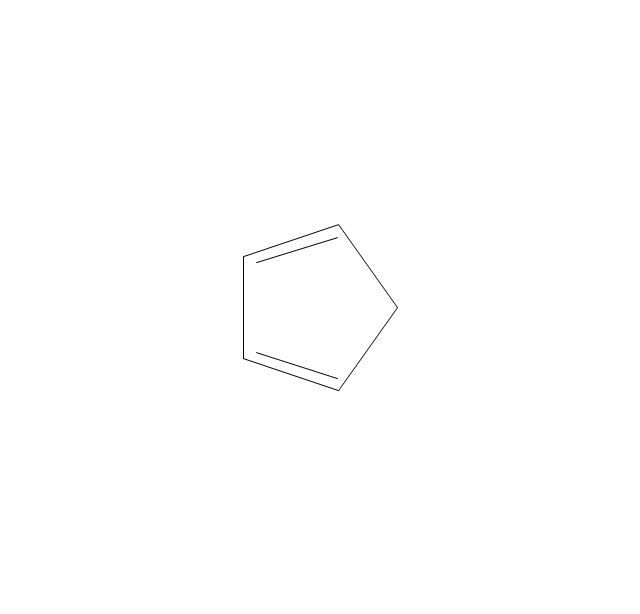
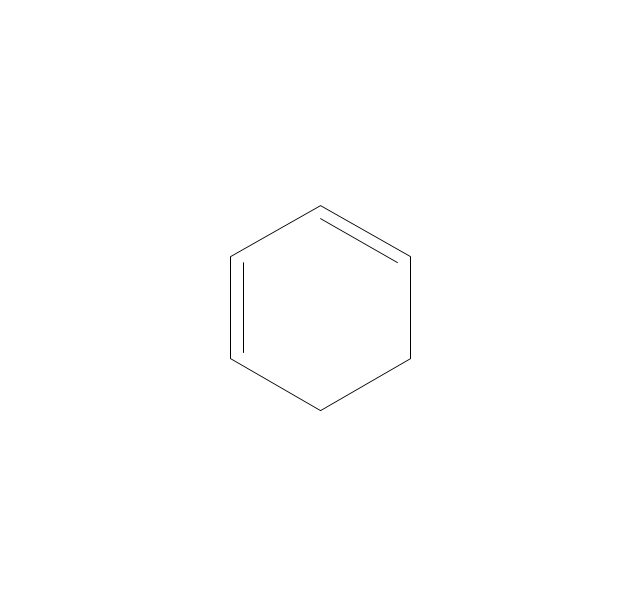
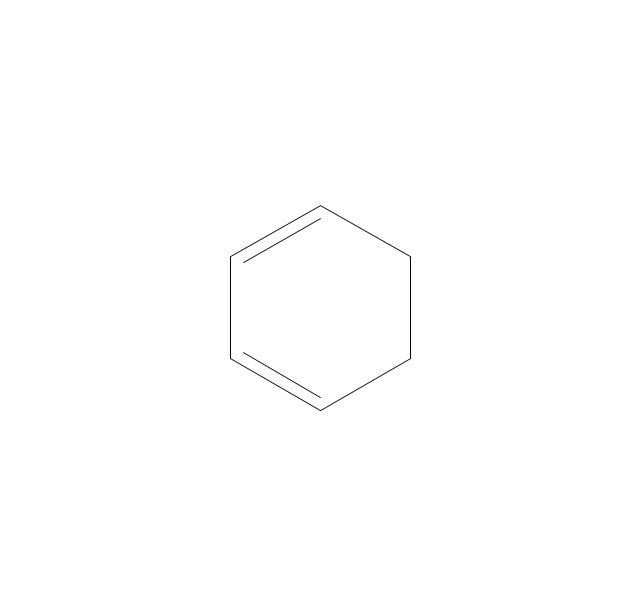
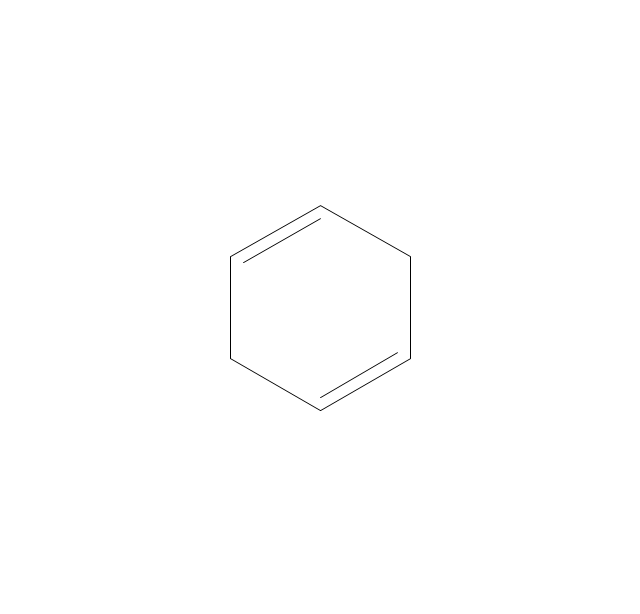
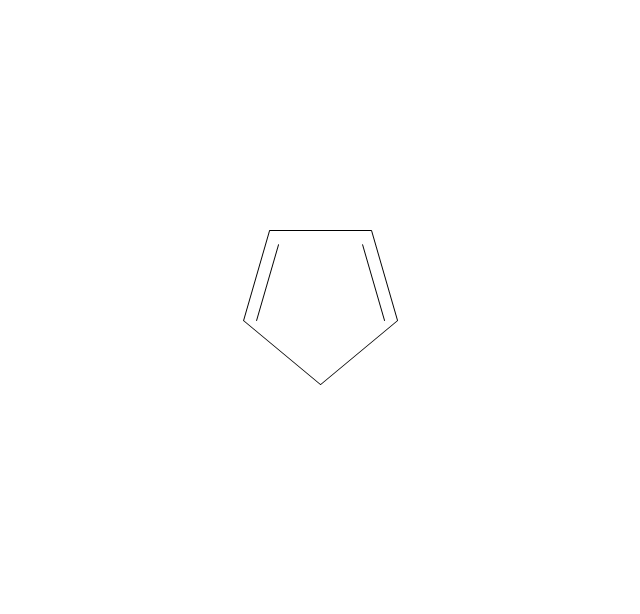
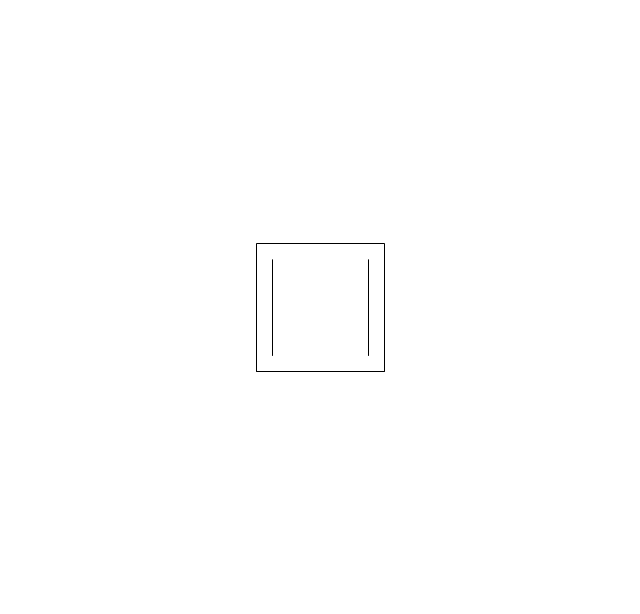
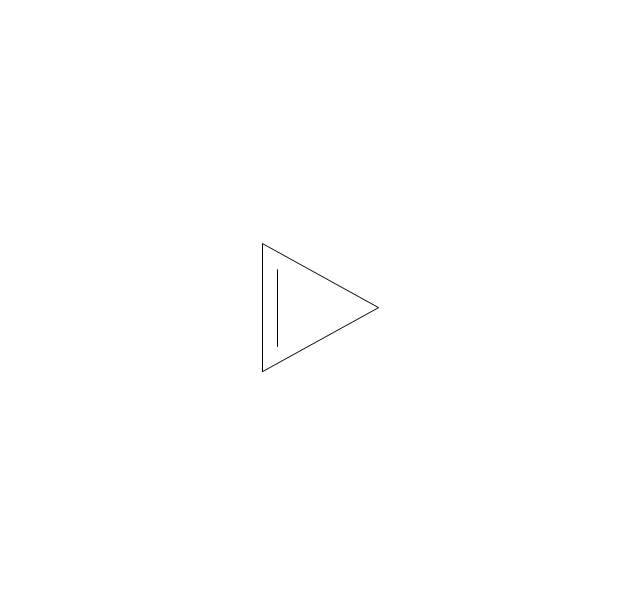
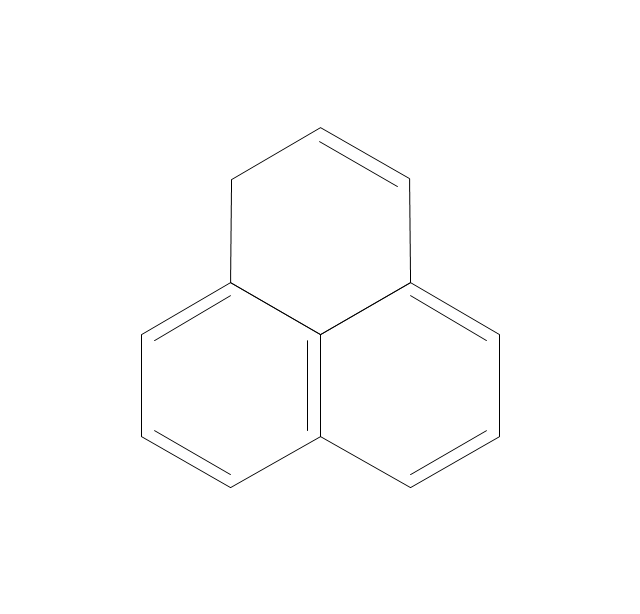
The vector stencils library "Aromatics" contains 23 symbols of aromatic rings for chemical drawing of molecular structural formulas and reaction mechanism schemes in organic chemistry.
"In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected by the stabilization of conjugation alone. ... Aromaticity can also be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-bonded to one another. These bonds may be seen as a hybrid of a single bond and a double bond, each bond in the ring identical to every other. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by Kekulé (see History section below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to give rise to six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization. ... Types of aromatic compounds. The overwhelming majority of aromatic compounds are compounds of carbon, but they need not be hydrocarbons. 1. Neutral homocyclics. Benzene, as well as most other annulenes (cyclodecapentaene excepted) with the formula CnHn where n is an even number, such as cyclotetradecaheptaene. 2. Heterocyclics. In heterocyclic aromatics (heteroaromats), one or more of the atoms in the aromatic ring is of an element other than carbon. This can lessen the ring's aromaticity, and thus (as in the case of furan) increase its reactivity. Other examples include pyridine, pyrazine, imidazole, pyrazole, oxazole, thiophene, and their benzannulated analogs (benzimidazole, for example). 3. Polycyclics. Polycyclic aromatic hydrocarbons are molecules containing two or more simple aromatic rings fused together by sharing two neighboring carbon atoms (see also simple aromatic rings). Examples are naphthalene, anthracene, and phenanthrene. 4. Substituted aromatics. Many chemical compounds are aromatic rings with other functional groups attached. Examples include trinitrotoluene (TNT), acetylsalicylic acid (aspirin), paracetamol, and the nucleotides of DNA. 5. Atypical aromatic compounds. Aromaticity is found in ions as well: the cyclopropenyl cation (2e system), the cyclopentadienyl anion (6e system), the tropylium ion (6e), and the cyclooctatetraene dianion (10e). Aromatic properties have been attributed to non-benzenoid compounds such as tropone. Aromatic properties are tested to the limit in a class of compounds called cyclophanes. A special case of aromaticity is found in homoaromaticity where conjugation is interrupted by a single sp³ hybridized carbon atom. When carbon in benzene is replaced by other elements in borabenzene, silabenzene, germanabenzene, stannabenzene, phosphorine or pyrylium salts the aromaticity is still retained. Aromaticity also occurs in compounds that are not carbon-based at all. Inorganic 6-membered-ring compounds analogous to benzene have been synthesized. Hexasilabenzene (Si6H6) and borazine (B3N3H6) are structurally analogous to benzene, with the carbon atoms replaced by another element or elements. In borazine, the boron and nitrogen atoms alternate around the ring." [Aromaticity. Wikipedia]
The organic compound structural formulas example "Aromatics - Vector stencils library" was created using the ConceptDraw PRO software extended with the Chemistry solution from the Science and Education area of ConceptDraw Solution Park.
"In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected by the stabilization of conjugation alone. ... Aromaticity can also be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-bonded to one another. These bonds may be seen as a hybrid of a single bond and a double bond, each bond in the ring identical to every other. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by Kekulé (see History section below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to give rise to six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization. ... Types of aromatic compounds. The overwhelming majority of aromatic compounds are compounds of carbon, but they need not be hydrocarbons. 1. Neutral homocyclics. Benzene, as well as most other annulenes (cyclodecapentaene excepted) with the formula CnHn where n is an even number, such as cyclotetradecaheptaene. 2. Heterocyclics. In heterocyclic aromatics (heteroaromats), one or more of the atoms in the aromatic ring is of an element other than carbon. This can lessen the ring's aromaticity, and thus (as in the case of furan) increase its reactivity. Other examples include pyridine, pyrazine, imidazole, pyrazole, oxazole, thiophene, and their benzannulated analogs (benzimidazole, for example). 3. Polycyclics. Polycyclic aromatic hydrocarbons are molecules containing two or more simple aromatic rings fused together by sharing two neighboring carbon atoms (see also simple aromatic rings). Examples are naphthalene, anthracene, and phenanthrene. 4. Substituted aromatics. Many chemical compounds are aromatic rings with other functional groups attached. Examples include trinitrotoluene (TNT), acetylsalicylic acid (aspirin), paracetamol, and the nucleotides of DNA. 5. Atypical aromatic compounds. Aromaticity is found in ions as well: the cyclopropenyl cation (2e system), the cyclopentadienyl anion (6e system), the tropylium ion (6e), and the cyclooctatetraene dianion (10e). Aromatic properties have been attributed to non-benzenoid compounds such as tropone. Aromatic properties are tested to the limit in a class of compounds called cyclophanes. A special case of aromaticity is found in homoaromaticity where conjugation is interrupted by a single sp³ hybridized carbon atom. When carbon in benzene is replaced by other elements in borabenzene, silabenzene, germanabenzene, stannabenzene, phosphorine or pyrylium salts the aromaticity is still retained. Aromaticity also occurs in compounds that are not carbon-based at all. Inorganic 6-membered-ring compounds analogous to benzene have been synthesized. Hexasilabenzene (Si6H6) and borazine (B3N3H6) are structurally analogous to benzene, with the carbon atoms replaced by another element or elements. In borazine, the boron and nitrogen atoms alternate around the ring." [Aromaticity. Wikipedia]
The organic compound structural formulas example "Aromatics - Vector stencils library" was created using the ConceptDraw PRO software extended with the Chemistry solution from the Science and Education area of ConceptDraw Solution Park.
The vector stencils library "Chemical elements" contains 118 icon symbols of chemical elements for drawing atoms, structural formulas and ball-and-stick models of inorganic and organic molecules and ions, and schemes of chemical reaction mechanisms.
"In chemistry, the ball-and-stick model is a molecular model of a chemical substance which is to display both the three-dimensional position of the atoms and the bonds between them. The atoms are typically represented by spheres, connected by rods which represent the bonds. Double and triple bonds are usually represented by two or three curved rods, respectively. In a good model, the angles between the rods should be the same as the angles between the bonds, and the distances between the centers of the spheres should be proportional to the distances between the corresponding atomic nuclei. The chemical element of each atom is often indicated by the sphere's color." [Ball-and-stick model. Wikipedia]
The chemical symbols example "Design elements - Chemical elements" was created using the ConceptDraw PRO software extended with the Chemistry solution from the Science and Education area of ConceptDraw Solution Park.
"In chemistry, the ball-and-stick model is a molecular model of a chemical substance which is to display both the three-dimensional position of the atoms and the bonds between them. The atoms are typically represented by spheres, connected by rods which represent the bonds. Double and triple bonds are usually represented by two or three curved rods, respectively. In a good model, the angles between the rods should be the same as the angles between the bonds, and the distances between the centers of the spheres should be proportional to the distances between the corresponding atomic nuclei. The chemical element of each atom is often indicated by the sphere's color." [Ball-and-stick model. Wikipedia]
The chemical symbols example "Design elements - Chemical elements" was created using the ConceptDraw PRO software extended with the Chemistry solution from the Science and Education area of ConceptDraw Solution Park.
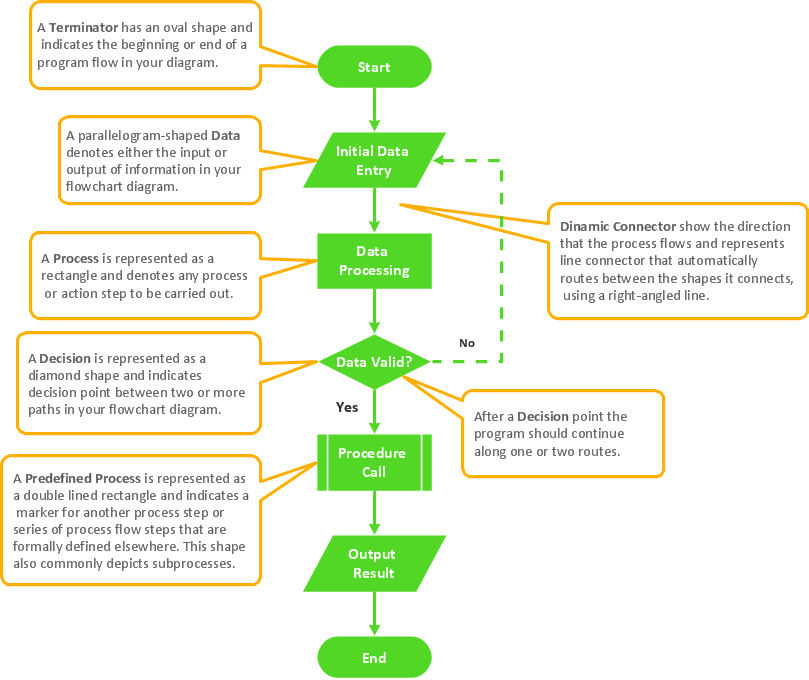
Flowchart Components
The ConceptDraw Flowchart component includes a set of samples and templates. This geathered as flowchart maker toolbox which focused on drawing flowcharts of any industry standards.The vector stencils library "Periodic table of chemical elements" contains 119 icon symbols of chemical elements for drawing Mendeleev's periodic table, chemical diagrams, infographics and illustrations.
"A chemical element is a pure chemical substance consisting of a single type of atom distinguished by its atomic number, which is the number of protons in its atomic nucleus. Elements are divided into metals, metalloids, and non-metals. Familiar examples of elements are carbon, nitrogen, oxygen (non-metals), silicon, arsenic (metalloids), aluminium, iron, copper, gold, mercury, and lead (metals).
The lightest chemical elements, including hydrogen, helium and smaller amounts of lithium, beryllium and boron, are thought to have been produced by various cosmic processes during the Big Bang and cosmic-ray spallation. Production of heavier elements, from carbon to the very heaviest elements, proceeded by stellar nucleosynthesis, and these were made available for later solar system and planetary formation by planetary nebulae and supernovae, which blast these elements into space. The high abundance of oxygen, silicon, and iron on Earth reflects their common production in such stars. While most elements are generally stable, a small amount of natural transformation of one element to another also occurs in the decay of radioactive elements as well as other natural nuclear processes." [Chemical element. Wikipedia]
The chemical symbols example "Design elements - Periodic table of chemical elements" was created using the ConceptDraw PRO software extended with the Chemistry solution from the Science and Education area of ConceptDraw Solution Park.
"A chemical element is a pure chemical substance consisting of a single type of atom distinguished by its atomic number, which is the number of protons in its atomic nucleus. Elements are divided into metals, metalloids, and non-metals. Familiar examples of elements are carbon, nitrogen, oxygen (non-metals), silicon, arsenic (metalloids), aluminium, iron, copper, gold, mercury, and lead (metals).
The lightest chemical elements, including hydrogen, helium and smaller amounts of lithium, beryllium and boron, are thought to have been produced by various cosmic processes during the Big Bang and cosmic-ray spallation. Production of heavier elements, from carbon to the very heaviest elements, proceeded by stellar nucleosynthesis, and these were made available for later solar system and planetary formation by planetary nebulae and supernovae, which blast these elements into space. The high abundance of oxygen, silicon, and iron on Earth reflects their common production in such stars. While most elements are generally stable, a small amount of natural transformation of one element to another also occurs in the decay of radioactive elements as well as other natural nuclear processes." [Chemical element. Wikipedia]
The chemical symbols example "Design elements - Periodic table of chemical elements" was created using the ConceptDraw PRO software extended with the Chemistry solution from the Science and Education area of ConceptDraw Solution Park.
 Chemistry
Chemistry
This solution extends ConceptDraw PRO software with samples, template and libraries of vector stencils for drawing the Chemistry Illustrations for science and education.
The vector stencils library "Resources and energy" contains 19 clipart images for drawing illustrations on resources and energy.
"Natural resources occur naturally within environments that exist relatively undisturbed by humanity, in a natural form. A natural resource is often characterized by amounts of biodiversity and geodiversity existent in various ecosystems.
Natural resources are derived from the environment. Some of them are essential for our survival while most are used for satisfying our wants. Natural resources may be further classified in different ways.
Natural resources are materials and components (something that can be used) that can be found within the environment. Every man-made product is composed of natural resources (at its fundamental level). A natural resource may exist as a separate entity such as fresh water, and air, as well as a living organism such as a fish, or it may exist in an alternate form which must be processed to obtain the resource such as metal ores, oil, and most forms of energy." [Natural resource. Wikipedia]
The clip art example "Resources and energy - Vector stencils library" was created in ConceptDraw PRO diagramming and vector drawing software using the Manufacturing and Maintenance solution from the Illustration area of ConceptDraw Solution Park.
"Natural resources occur naturally within environments that exist relatively undisturbed by humanity, in a natural form. A natural resource is often characterized by amounts of biodiversity and geodiversity existent in various ecosystems.
Natural resources are derived from the environment. Some of them are essential for our survival while most are used for satisfying our wants. Natural resources may be further classified in different ways.
Natural resources are materials and components (something that can be used) that can be found within the environment. Every man-made product is composed of natural resources (at its fundamental level). A natural resource may exist as a separate entity such as fresh water, and air, as well as a living organism such as a fish, or it may exist in an alternate form which must be processed to obtain the resource such as metal ores, oil, and most forms of energy." [Natural resource. Wikipedia]
The clip art example "Resources and energy - Vector stencils library" was created in ConceptDraw PRO diagramming and vector drawing software using the Manufacturing and Maintenance solution from the Illustration area of ConceptDraw Solution Park.
The vector stencils library "Education pictograms" contains 128 education pictograms. Use this flat icon set to design your educational infogram in ConceptDraw PRO diagramming and vector drawing software.
The vector stencils library "Education pictograms" is included in the Education Infographics solution from the Business Infographics area of ConceptDraw Solution Park.
The vector stencils library "Education pictograms" is included in the Education Infographics solution from the Business Infographics area of ConceptDraw Solution Park.
Chemistry Drawings
ConceptDraw PRO diagramming and vector drawing software extended with Chemistry solution from the Science and Education area is a powerful chemistry drawing software that is ideal for quick and easy designing of various: chemistry drawings, scientific and educational chemistry illustrations, schemes and diagrams of chemical and biological lab set-ups, images with chemical formulas, molecular structures, chemical reaction schemes, schemes of labware, that can be then successfully used in the field of science and education, on various conferences, and so on.Design Element: Basic Network for Network Diagrams
ConceptDraw PRO is perfect for software designers and software developers who need to draw Basic Network Diagrams.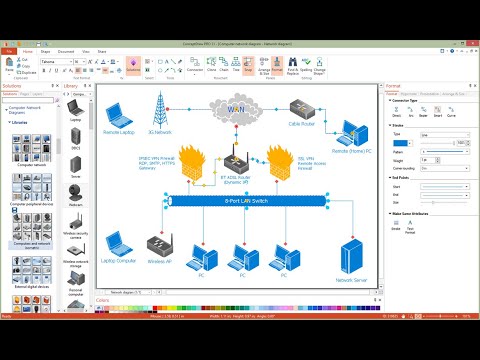
- Design elements - Periodic table of chemical elements | Education ...
- Chemical elements - Vector stencils library | Design elements ...
- Chemical elements - Vector stencils library
- Aromatics - Vector stencils library | Aromatics - Vector stencils library ...
- Design elements - Chemical elements | Design elements - Periodic ...
- Design elements - Nuclear physics | Nuclear physics - Vector ...
- Stars and planets - Vector stencils library | Design elements ...
- Aromatics - Vector stencils library | Design elements - Aromatic ...
- Design elements - Aromatic hydrocarbons (arenes) | Aromatics ...
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)
-chemical-elements---vector-stencils-library.png--diagram-flowchart-example.png)

































































































.png)